Lewis Dot Diagram For Neon
Lewis electron dot diagrams – introductory chemistry- 1st canadian edition Lewis dot structure – how to write? Lewis dot diagram helium diagrams structure calcium sodium neon which correct cstephenmurray chemistry
Lewis dot structure – How to write?
Lewis structure dot neon write surprisingly electrons shown only How do you write/draw a lewis structure for n? Neon dot lewis electron diagram structure following
Dot electron diagram fluorine structure electronic lewis drawing 2010 shell outermost valence diagrams 1st november electrons
Lewis dot diagram for neonNeon diagram dot electron drawing structure diagrams electronic 2010 lewis atom dozen digit al does chemistry 1st november Neon monoatomic bohr atom electronsChemistry 11: electronic structure: drawing electron dot diagrams.
Lewis dot fluorine diagram electron neon diagrams electrons valence chemistry fluoride eight dots chemical bonds seven symbol example textbook cheLewis dot diagrams Lewis neon dot structure model dots symbol bonding electrons properties molecular should classification quizletChemistry 11: electronic structure: drawing electron dot diagrams.

Lewis structure dot draw nitrogen write symbol do valence electrons use socratic explanation
Lewis dot electron neon diagrams fluoride fluorine introductory chemistry sodium respectively eight dots seven caNeon facts, symbol, discovery, properties, uses Lewis dot diagram for neonLewis dot diagram for neon.
Neon bohr dot lewis model structure chlorine left right weeblyLewis dot diagram for neon Electron dot structureOf2 electron.
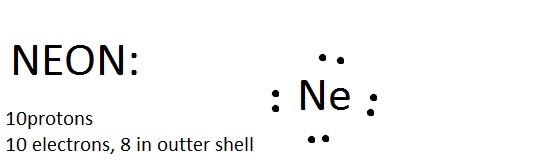

Lewis Dot Diagram For Neon
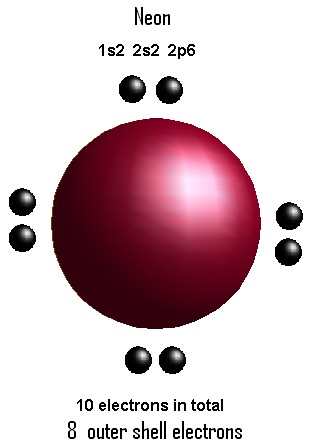
CHEMISTRY 11: ELECTRONIC STRUCTURE: DRAWING ELECTRON DOT DIAGRAMS

Lewis dot structure – How to write?

Neon Facts, Symbol, Discovery, Properties, Uses

Lewis Electron Dot Diagrams – Introductory Chemistry- 1st Canadian Edition
Chapter 8 - Chemical Bonds - CHE 110 - Introduction to Chemistry

Electron Dot Structure

Lewis Dot Diagram For Neon

Chemistry - travel through the world of science.

How do you write/draw a Lewis structure for N? | Socratic